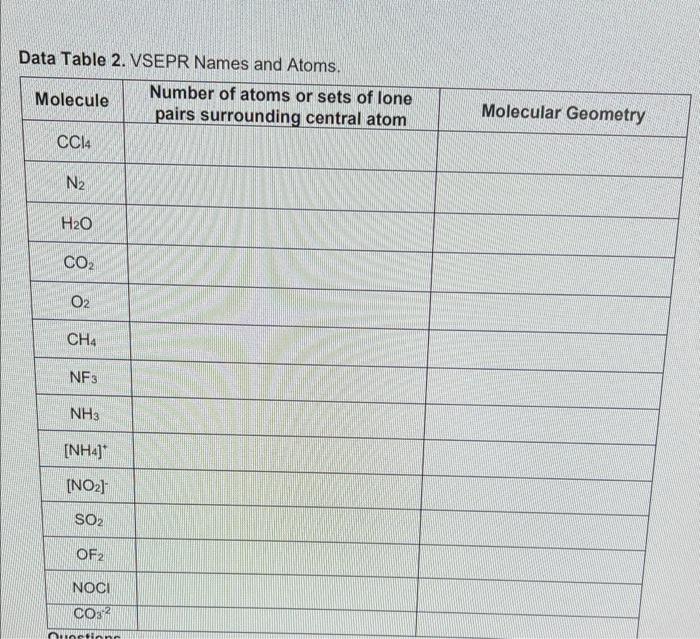
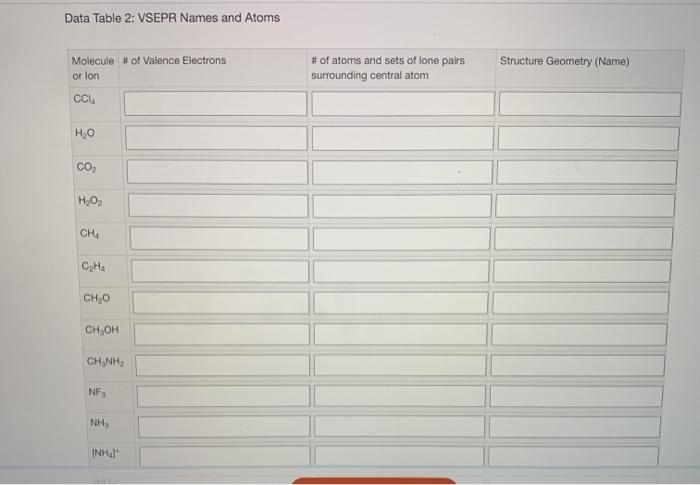
Data table 2 vsepr names and atoms – Embark on an enlightening journey into the realm of chemistry as we delve into Data Table 2, a pivotal tool in determining VSEPR names and unraveling the intricate structures of atoms. This comprehensive guide will illuminate the significance of this table, empowering you to decipher molecular geometries and predict chemical properties with unparalleled precision.
Data Table 2 stands as a cornerstone in the study of molecular structures, providing a wealth of information about elements and their electron configurations. Through its meticulous organization, we can uncover the fundamental principles that govern the shapes and properties of molecules.
Introduction to Data Table 2: Data Table 2 Vsepr Names And Atoms

Data Table 2 is a valuable resource in chemistry that provides crucial information for determining VSEPR names and atomic structures. It is a comprehensive table that lists the atomic number, element symbol, and electron configuration of various elements.
The electron configuration of an element, represented by its valence electrons, plays a vital role in determining the geometry of a molecule. Data Table 2 allows chemists to quickly and easily access this information, making it an indispensable tool for predicting molecular shapes.
Key Elements of Data Table 2, Data table 2 vsepr names and atoms
Data Table 2 consists of several columns and rows, each providing specific information about the elements:
- Atomic Number:The number of protons in the nucleus of an atom, represented by the symbol Z.
- Element Symbol:The one- or two-letter abbreviation that represents an element, e.g., H for hydrogen.
- Electron Configuration:The distribution of electrons in the atomic orbitals, expressed using the Aufbau principle.
Using Data Table 2 to Determine VSEPR Names
VSEPR theory (Valence Shell Electron Pair Repulsion) predicts the molecular geometry based on the number of valence electrons and the type of hybridization. To use Data Table 2 to determine VSEPR names, follow these steps:
- Identify the element in the molecule and locate its corresponding row in the table.
- Determine the number of valence electrons by looking at the electron configuration in the last column.
- Use the valence electrons to predict the hybridization and molecular geometry using VSEPR theory.
Examples of VSEPR Names and Molecular Geometries
The following table provides examples of VSEPR names and their corresponding molecular geometries:
| VSEPR Name | Molecular Geometry | Lewis Structure | Electron Configuration |
|---|---|---|---|
| AX2 | Linear | :A-X-X: | AX2e– |
| AX3 | Trigonal Planar | :A-X:|:X-X: | AX3e– |
| AX4 | Tetrahedral | :A:|X-X-X-X | AX4e– |
Applications of VSEPR Theory
VSEPR theory has numerous practical applications in chemistry, including:
- Predicting the molecular geometry and shape of molecules.
- Determining the polarity and reactivity of molecules.
- Understanding the electronic structure and bonding in molecules.
FAQ Corner
What is the purpose of Data Table 2?
Data Table 2 provides essential information about elements, including their atomic number, element symbol, and electron configuration. This data is crucial for determining VSEPR names and understanding atomic structures.
How do I use Data Table 2 to determine VSEPR names?
To determine VSEPR names using Data Table 2, identify the element’s group number and period. This information will provide the number of valence electrons, which determines the molecular geometry and VSEPR name.
What are the practical applications of VSEPR theory?
VSEPR theory finds widespread applications in chemistry, including predicting molecular shapes, understanding chemical bonding, and designing new materials. It provides valuable insights into the properties and reactivity of molecules.