Shall we dance classifying types of chemical reactions – Shall we dance? Classifying types of chemical reactions is an engaging journey into the fascinating world of chemistry. From the simplest combination reactions to the more complex combustion reactions, understanding these reactions provides a deeper appreciation of the chemical processes that shape our world.
This comprehensive guide will explore the different types of chemical reactions, their characteristics, and the factors that influence their rates. We will also delve into the practical applications of chemical reactions in various fields, from industry to medicine, and examine their importance in modern society.
Types of Chemical Reactions
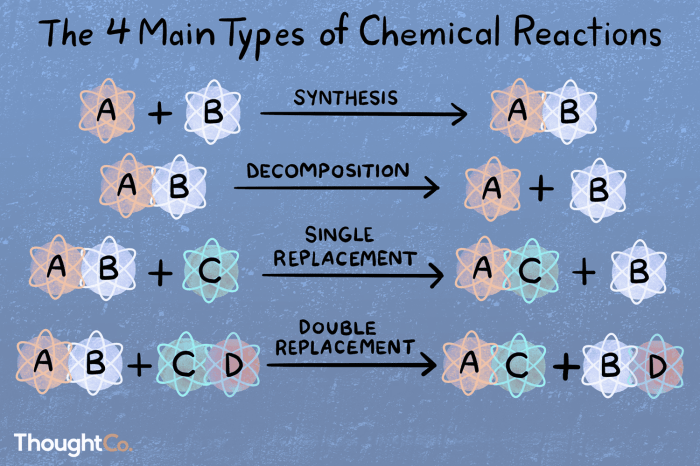
Chemical reactions are processes that involve the transformation of one set of chemical substances into another. There are five main types of chemical reactions:
Combination Reactions
- Two or more substances combine to form a single product.
- Example: 2H 2+ O 2→ 2H 2O
Decomposition Reactions, Shall we dance classifying types of chemical reactions
- A single compound breaks down into two or more simpler substances.
- Example: 2H 2O → 2H 2+ O 2
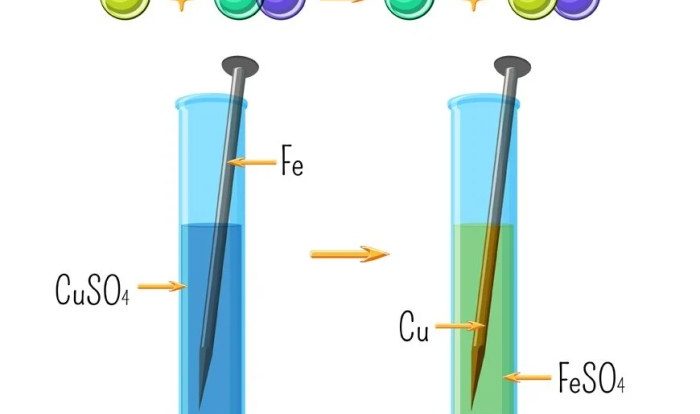
Single Displacement Reactions
- An element replaces another element in a compound.
- Example: Fe + 2HCl → FeCl 2+ H 2
Double Displacement Reactions
- Two compounds exchange ions to form two new compounds.
- Example: NaCl + AgNO 3→ NaNO 3+ AgCl
Combustion Reactions
- A substance reacts with oxygen, usually producing heat and light.
- Example: CH 4+ 2O 2→ CO 2+ 2H 2O
Factors Affecting Chemical Reactions
Several factors can affect the rate of a chemical reaction:
Temperature
- Increasing temperature generally increases the reaction rate.
- Example: Cooking food at a higher temperature speeds up the chemical reactions that break down the food.
Concentration
- Increasing the concentration of reactants increases the reaction rate.
- Example: Adding more acid to a solution speeds up the reaction between the acid and a metal.
Surface Area
- Increasing the surface area of reactants increases the reaction rate.
- Example: Grinding a solid reactant into a powder increases its surface area and speeds up the reaction.
Catalyst
- A catalyst is a substance that speeds up a reaction without being consumed.
- Example: Enzymes in the human body act as catalysts to speed up biochemical reactions.
Applications of Chemical Reactions: Shall We Dance Classifying Types Of Chemical Reactions
Chemical reactions are used in a wide variety of applications:
Industry
- Producing chemicals, plastics, and other materials.
- Example: The Haber process is used to produce ammonia, which is essential for fertilizer production.
Medicine
- Developing new drugs and treatments.
- Example: Chemotherapy drugs use chemical reactions to kill cancer cells.
Everyday Life
- Cooking, cleaning, and digestion.
- Example: The reaction between baking soda and vinegar creates carbon dioxide gas, which makes cakes rise.
Balancing Chemical Equations

Balancing chemical equations is important to ensure that the number of atoms of each element is the same on both sides of the equation.
Steps to Balance Chemical Equations
- Write the unbalanced equation.
- Count the number of atoms of each element on both sides.
- Add coefficients to the reactants and products to balance the atoms.
- Check that the equation is balanced.
| Unbalanced Equation | Balanced Equation |
|---|---|
| 2H2 + O2 → H2O | 2H2 + O2 → 2H2O |
| Fe + 2HCl → FeCl2 + H2 | Fe + 2HCl → FeCl2 + H2 |
Chemical Reactions in Everyday Life
Chemical reactions are involved in many everyday activities:
Cooking
- The Maillard reaction creates the brown color and flavor of roasted meats.
Cleaning
- Bleach uses a chemical reaction to remove stains from clothes.
Digestion
- Enzymes in the stomach break down food into smaller molecules.
FAQ Overview
What are the five main types of chemical reactions?
The five main types of chemical reactions are combination, decomposition, single displacement, double displacement, and combustion reactions.
What factors affect the rate of chemical reactions?
The rate of chemical reactions is affected by temperature, concentration, surface area, and the presence of a catalyst.
What are some examples of chemical reactions in everyday life?
Examples of chemical reactions in everyday life include cooking, cleaning, and digestion.