Draw cis-1-ethyl-2-isopropylcyclohexane in its lowest energy conformation – In this exploration, we delve into the captivating realm of conformational analysis, drawing cis-1-ethyl-2-isopropylcyclohexane in its lowest energy conformation. This molecular journey unravels the intricacies of structural features, conformational analysis, and the factors that govern the stability of this intriguing molecule.
The molecular structure of cis-1-ethyl-2-isopropylcyclohexane, with its ethyl and isopropyl groups strategically positioned relative to the cyclohexane ring, sets the stage for a captivating analysis of its conformational landscape. As we embark on this exploration, we will uncover the factors that shape the molecule’s preferred conformation and its implications in various scientific disciplines.
Structural Features
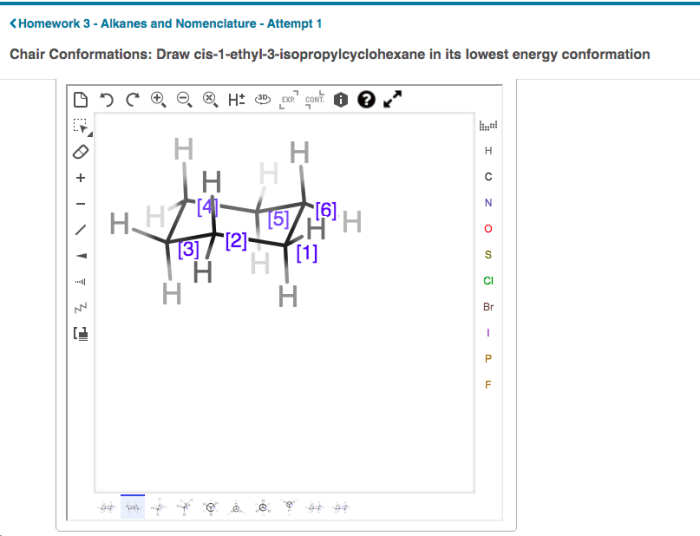
Cis-1-ethyl-2-isopropylcyclohexane is a cyclic hydrocarbon molecule with a six-membered cyclohexane ring and two alkyl substituents: an ethyl group (C 2H 5) and an isopropyl group (C 3H 7). The ethyl group is attached to carbon 1 of the cyclohexane ring, while the isopropyl group is attached to carbon 2. The molecule can be represented using a skeletal formula as follows:
Conformational Analysis
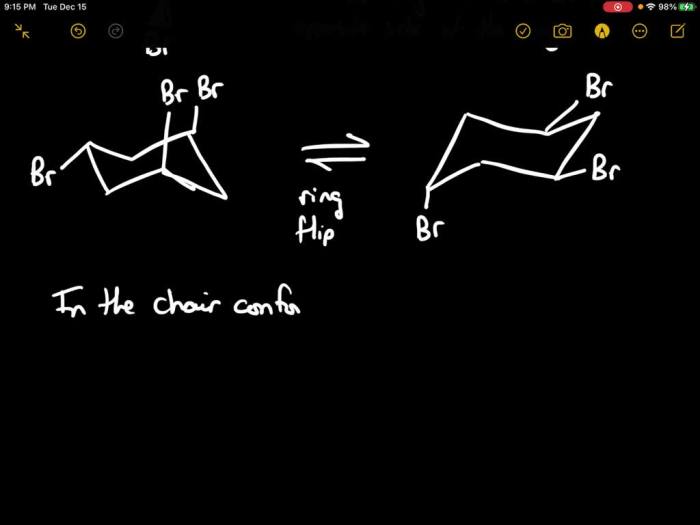
Conformational analysis is the study of the different three-dimensional arrangements of atoms in a molecule. For cis-1-ethyl-2-isopropylcyclohexane, there are two possible conformations:
- Axial-axial conformation:In this conformation, both the ethyl and isopropyl groups are oriented in the same direction relative to the cyclohexane ring. This conformation is less stable due to steric hindrance between the two alkyl groups.
- Equatorial-equatorial conformation:In this conformation, both the ethyl and isopropyl groups are oriented away from each other, minimizing steric hindrance. This conformation is more stable than the axial-axial conformation.
The equatorial-equatorial conformation is the lowest energy conformation for cis-1-ethyl-2-isopropylcyclohexane.
Factors Influencing Conformation
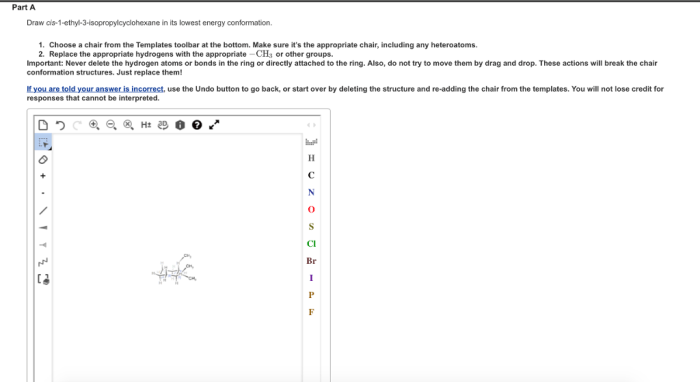
The conformation of cis-1-ethyl-2-isopropylcyclohexane is influenced by several factors, including:
- Steric interactions:The steric hindrance between the ethyl and isopropyl groups is the primary factor that determines the stability of the different conformations. The equatorial-equatorial conformation minimizes steric hindrance and is therefore more stable.
- Van der Waals forces:Van der Waals forces are attractive forces between nonpolar molecules. In cis-1-ethyl-2-isopropylcyclohexane, the equatorial-equatorial conformation allows for more van der Waals interactions between the alkyl groups and the cyclohexane ring, which contributes to its stability.
- Dipole-dipole interactions:The ethyl and isopropyl groups have slight dipole moments. In the equatorial-equatorial conformation, the dipole moments of the two alkyl groups cancel each other out, resulting in a more stable conformation.
Applications
Understanding the conformational analysis of cis-1-ethyl-2-isopropylcyclohexane has applications in various fields, including:
- Drug design:The conformation of a drug molecule can affect its binding affinity to a target protein. By understanding the conformational preferences of a drug molecule, researchers can design drugs that are more likely to bind to their target and have the desired therapeutic effect.
- Materials science:The conformation of polymer molecules can affect the properties of the material. By understanding the conformational preferences of a polymer molecule, researchers can design materials with specific properties, such as strength, flexibility, and thermal stability.
- Catalysis:The conformation of a catalyst molecule can affect its activity and selectivity. By understanding the conformational preferences of a catalyst molecule, researchers can design catalysts that are more efficient and selective for specific reactions.
FAQ Insights: Draw Cis-1-ethyl-2-isopropylcyclohexane In Its Lowest Energy Conformation
What is the significance of conformational analysis in understanding the properties of molecules?
Conformational analysis provides crucial insights into the three-dimensional arrangement of atoms within a molecule, revealing how different conformations can influence physical and chemical properties, such as reactivity, solubility, and biological activity.
How do steric interactions impact the conformation of cis-1-ethyl-2-isopropylcyclohexane?
Steric interactions between the bulky ethyl and isopropyl groups play a significant role in determining the preferred conformation of the molecule, as they hinder the free rotation of the cyclohexane ring and favor conformations that minimize these interactions.
What are the potential applications of understanding the conformational analysis of cis-1-ethyl-2-isopropylcyclohexane?
Understanding the conformational analysis of cis-1-ethyl-2-isopropylcyclohexane has implications in drug design, materials science, and catalysis. By manipulating the conformation of molecules, scientists can tailor their properties for specific applications, such as enhancing drug efficacy, improving material performance, and optimizing catalytic activity.